
Welcome to the Research Institute of Healthcare Sciences (RIHS)
RIHS fosters and facilitates research in laboratory, translational & clinical science and community & clinical practice to understand human disease at the cellular, individual and populations levels and to use this knowledge to improve survivorship and quality of life and care.
Building dynamic multi-disciplinary research teams and applying collaborative holistic strategies to tackle complex healthcare challenges enables us to translate laboratory and practice-based research quickly and effectively into clinical benefit.
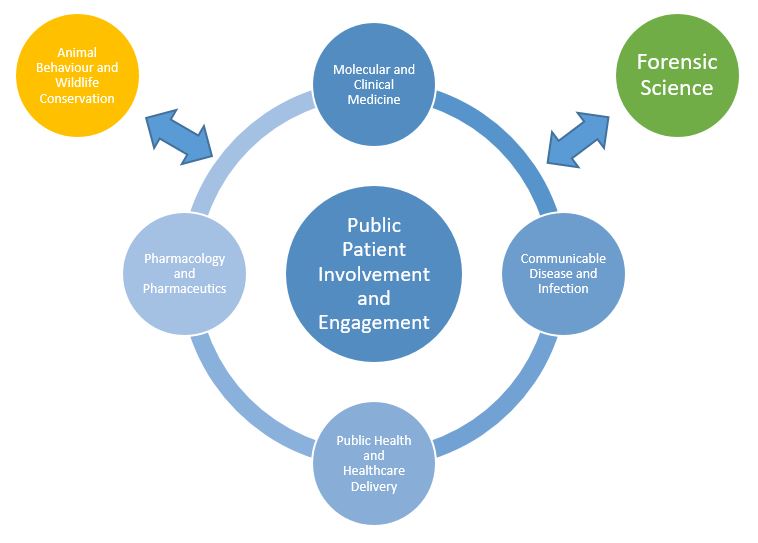
Our research themes:
Spotlight on our research
A team of new professors in Clinical Practice and Medicine have been appointed by the University of Wolverhampton and The Royal Wolverhampton NHS Trust to drive forward medical research in the region - see https://www.wlv.ac.uk/research/about-our-research/impact/clinical-research/.
The STORK Collaborative is a network of interested health care teams working towards reducing risks for newborn and infant mortality though parent, carer and family education and empowerment, using the STORK Programme, in the West Midlands.
About RIHS
For REF 2021 we submitted four Impact Case Studies under Unit of Assessment 3 (Allied Health Professions, Dentistry, Nursing and Pharmacy). These spanned a range of impacts, from contributing to health policy and guidelines, to evidence of direct benefits to patients, and even as far as impacts on art and culture.
Specifically the Impact Case Studies covered the following topic areas:
- In partnership with colleagues from the Centre for Psychological Research, utilising research research on eating behaviour and digestive disorders, we informed national health policy and to underpin the ‘Living in Silence project, which uses various art forms to better inform those from South Asian communities who are living with Inflammatory Bowel Disease (IBD).
- Using our knowledge on working with bereaved families to inform policy and guidance around organ donation across various NHS trusts, as well as large independent organisations.
- In partnership with colleagues from the Sport and Physical Activity Research Centre, we were able to show how exercise can be effectively utilised to benefit the health of cancer patients. Impacts ranged from underpinning the health programme Action Health based from Dudley Group NHS Foundation Trust, to informing best practice documents of various large cancer organisations across the world.
- Our research on clinical communication with patients was used to inform a large variety of health policy documents from key national and international organisations.
The University has invested heavily in the recruitment of research active staff and the provision of high quality research facilities, including a £1.6m investment in provide world-class biomedical science laboratory accommodation and new build support for psychology and exercise physiology research.
There is a distinctive emphasis trying to ensure that the findings of academic research are quickly and effectively translated into clinical practice. And there is close collaboration with local NHS Trusts.
Self-funded PhD Projects
The self-funded PhD projects are listed below for Biological Sciences, Biomedical Sciences, Chemistry, Molecular Biology, Pharmacy and Botany.
Choose one project and specify the reference number in your application form.
Biological Sciences Self-funded PhD Projects (RIHS)
Reference: 1/TB/24/RIHS/BioS
Self funded PhD Postgraduate research in Biological Sciences
Project details
Acacia senegal is a species of legume, native to the Sahelian region of Africa. When wounded, mature trees of this species secrete a valuable plant gum exudate (termed gum Arabic) to seal the damaged region of the plant. This gum is comprised of a variety of macromolecules commonly associated with the plant cell wall. This exudate has been harvested on a commercial basis for thousands of years and is widely used in the food industry.
The chemical, biochemical and biophysical/physicochemical properties of gum Arabic have been intensively studied for over sixty years. However, little work has been conducted on the cellular and molecular wound response which is responsible for the biosynthesis of the gum. The objectives of the proposed project, therefore, would be to investigate the molecular structure and composition of the plant cell wall in seedlings of this species (Dr Baldwin: University of Wolverhampton), in conjunction with chemical and biochemical analyses of gum samples (harvested from the seedlings’ parent plants) (Professor Williams and Dr Jixin Yang: Glyndwr University). In addition, the proposed project will also include a transcriptomic study of the molecular basis of gummosis that will be performed in the laboratory of Professor Paul Dupree (University of Cambridge).
Methodology
The proposed project will consist of four main areas of study: 1) analytical chemistry/biochemistry 2) light level and transmission electron microscope level immunocytochemistry 3) molecular analyses of gum synthesis.
The initial phase of the project will mainly focus on chemical and biochemical analyses of gum Arabic samples. The bulk of this work will be performed at Glyndwr University in collaboration with Professor Williams and Dr Yang. This will involve the determination of the sugar composition using HPLC, and molecular mass distribution using GPC coupled to MALLS/RI/UV detectors, FTIR etc. The biochemical studies will include determination of total nitrogen content, monosaccharide and amino acid composition analyses, SDS-page, Western blots and immuno-dot blots.
At the same time, Acacia seeds obtained from Nigeria (Rubber Research Institute) will be germinated and the resultant seedlings grown, in the glasshouse facility at UoW. At six months post germination, plant material from the stem and branches from several of the seedlings will be harvested, fixed and embedded in L.R. white resin. The resin embedded samples will subsequently be sectioned and screened against a panel of anti-cell wall antibodies using light level and transmission electron microscope level immunocytochemistry, to investigate the structure and molecular composition of their cell walls.
The final phase of the study will involve an investigation of gene expression related to gum synthesis (using a transcriptomics approach) will be performed on RNA extracted from the A.senegal seedlings, in collaboration with Professor Paul Dupree based at the University of Cambridge.
Supervisory Team
Dr Timothy Baldwin (Reader in Plant Cell biology)
Professor Paul Dupree (University of Cambridge)
Professor Peter Williams & Dr Jixin Yang (Glyndwr University)
For more information: For an informal discussion please email Dr Timothy Baldwin - T.Baldwin@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biological Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biological-sciences/
and click on Apply Now.
Reference: 2/PB/24/RIHS/BioS
Self funded PhD Postgraduate research in Biological Sciences
Project details
The human gastric wall contains a variety of receptors detecting, among other things, temperature, stretch and osmolarity. These are linked to afferents in the autonomic nervous system and cause a variety of systemic consequences, including modulation of cardiac autonomic control resulting in acute changes in heart rate, cardiac output and blood pressure. We have already shown that stimulation of gastric TRPM8 cold receptors by menthol results in an acute increase in cardiac parasympathetic tone and concomitant reduction in heart rate and blood pressure, counteracting the increase in cardiac sympathetic activity caused by gastric stretch via TRPV4 receptors when both receptors are stimulated by a cold meal (Kazadi et al., 2018. https://doi.org/10.1113/EP087058). Using analysis of respiratory sinus arrhythmia as a measure of cardiac parasympathetic tone (Task Force of the European Society of Cardiology et al., 1996. https://doi.org/10.1161/01.CIR.93.5.1043) and QTc analysis of ECG as a measure of cardiac sympathetic tone, you will further investigate the effects of other gastric receptor activation on acute cardiac autonomic control. Research will be undertaken in the physiology research laboratory on the Wolverhampton City campus. This research will lead to the submission of a thesis for the award of Doctor of Philosophy (PhD) and will be published at research conferences and in scientific journals as the data warrant.
Supervisory Team
Dr Paul Barrow, Senior Lecturer in Physiology and Pharmacology
For more information: For an informal discussion please email Dr Paul Barrow - p.a.barrow@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biological Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biological-sciences/
and click on Apply Now.
Reference: 18/SV/24/RIHS/BioS
Self funded PhD Postgraduate research in Biological Sciences
Project detail
Modern zoos are involved in captive breeding and reintroduction programmes and, thus, play a pivotal role in fighting biodiversity loss. However, several endangered animal species are currently showing a low success rate in captive breeding, which impair them from possibly serving as a buffer against extinction. In the zoo environment, the lack of stimuli and the repetitive routine can lead to boredom and to the display of stereotypic behaviours, as well as endocrinological dysfunction, which may be linked with decreased reproductive fitness of captive populations. Nevertheless, captivity is a human-controlled environment and, therefore, it is possible to enhance captive breeding via evidence-based facilitation of reproductive behaviours and environmental enrichments.
This project aims to develop novel techniques for captive animal welfare based on the supervisory team experience in olfactory and acoustic communication. We will initially focus our research on critically endangered primate species that can be difficult to breed and maintain in captivity (i.e., lemurs and tamarins). The PhD student will gather behavioural data and samples for analysis, focussing on two main areas of research for commercialisation:
1) Olfactory enrichment: the PhD student will study the scent-marking behaviour of primate groups and, with the assistance of zoo staff, will gather olfactory samples for laboratory analysis. The chemical profiles of the samples will be examined using solid-phase microextraction and gas chromatography-mass spectrometry techniques. This will allow for the identification of specific compounds associated with the biology and behaviour of the individual primates. Compounds of interest (e.g., those relating to female fertility or affiliative behaviour) will then be isolated, synthesised, and used in olfactory enrichment experiments – i.e., presented to other individuals to evaluate their responses. Such approaches have been proven to improve welfare and reproductive success in domestic animal species (e.g., the Feliway© cat calming pheromones on sale to the public), but little is known about this in non-human primates or wildlife more broadly.
2) Acoustic communication: the PhD student will study the behaviour of the animals and will simultaneously record all vocalisations using active and passive acoustic recording. This will allow us to analyse individual calls associated with the biology and behaviour of specific individuals. Acoustic data will be analysed, and machine learning methods will be developed to automatically detect and classify calls. We will analyse calls that are related to positive and negative experiences (e.g., feeding vs. aggressive interactions) to understand how acoustic analysis could be used as a novel welfare monitoring technique. Such methods have previously been used with domestic animals (e.g., chickens), but have not yet been implemented in wildlife.
The expected outputs of this project will include:
- Training for the PhD student, who will work with us and learn new field and lab skills.
- Data towards the development of two different technical approaches to animal welfare and conservation (olfactory enrichment and acoustic monitoring).
- Data for high-quality peer-reviewed publications, presenting our findings and supporting an impact case study, currently being developed by Dr Vaglio on the theme of animal welfare and conservation.
Supervisory Team
Dr Stefano Vaglio (Reader in Animal Behaviour)
Dr Colin Dubreuil (Lecturer in Conservation Biology)
Dr Andrew Gascoigne (Senior Lecturer Mathematics & Computer Science)
Dr Jacob Dunn (Associate Professor in Evolutionary Biology – Anglia Ruskin University)
For more information: For an informal discussion please email Dr Stefano Vaglio - S.Vaglio@wlv.ac.uk
Reference: 32/NK/24/RIHS/BioS
Self-funded PhD Postgraduate research in Biological Sciences
Project details
Amphibians represent the most threatened vertebrate group, with 41% of species being in danger of extinction (IUCN, 2023). The common toad (Bufo bufo) native to the UK, has been reportedly facing major declines within the last decade (Wilkinson, 2019). Although there are potentially numerous, non-mutually exclusive causes of these declines, one particular concern is the effect of microplastics (MP) (Boyero et al., 2020). Over the last decades plastic production and use have increased exponentially, resulting in large quantities of plastic waste (Geyer et al., 2017). Plastic waste that ends up in aquatic environments can be broken down into smaller particles by chemical and photochemical reactions (Güven et al., 2017). Frogs can ingest MPs directly or via prey, these accumulate in the tissues and can impair survival, body condition and function (Boyero et al., 2020) However, little is known about the multi-stressor effect of MPs interacting with gastrointestinal parasites. The presence of these parasites is predicted to influence the retention of MPs in the gastrointestinal tract by increasing the surface area for adhesion and retention (ex., Hernandez-Milian et al., 2019). Additionally, they can also act as a vector for other contaminants, such as pathogens increasing transmission between individuals (Gkoutselis et al., 2021). MPs are ubiquitous, abundant and persistent over time, which highlights the need for understanding these multi-stressor effects in declining populations of common toads.
Aim
- To investigate the effect of microplastic (MP) ingestion and accumulation on common toad body condition and gastrointestinal parasite composition.
Objectives
- To assess MPs in the freshwater breeding habitats of common toads in urban and rural areas.
- To characterise the type of MPs present in the gastrointestinal tract of common toads collected from these breeding sites.
- To identify the gastrointestinal parasite composition of common toads from different breeding sites.
- To determine individual common toad body condition in relation to type and number of MPs identified in the gastrointestinal tract.
- To determine individual common toad parasite composition in relation to type and number of MPs identified in the gastrointestinal tract.
- Thorough analysis of the data and literature leading to publication.
References
Boyero, L., López-Rojo, N., Bosch, J., Alonso, A., Correa-Araneda, F. and Pérez, J., 2020. Microplastics impair amphibian survival, body condition and function. Chemosphere, 244, p.125500.
Geyer, R., Jambeck, J.R. and Law, K.L., 2017. Production, use, and fate of all plastics ever made. Science advances, 3(7), p.e1700782.
Gkoutselis, G., Rohrbach, S., Harjes, J., Obst, M., Brachmann, A., Horn, M.A. and Rambold, G., 2021. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Scientific Reports, 11(1), p.13214.
Güven, O., Gökdağ, K., Jovanović, B. and Kıdeyş, A.E., 2017. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environmental pollution, 223, pp.286-294.
Hernandez-Milian, G., Lusher, A., MacGabban, S. and Rogan, E., 2019. Microplastics in grey seal (Halichoerus grypus) intestines: Are they associated with parasite aggregations?. Marine Pollution Bulletin, 146, pp.349-354.
Wilkinson, J. 2019. Interviewed by IUCN SSC Amphibian Specialist Group. September 2019, UK.
Supervisory Team
Dr Natasha Kruger (Lecturer in Animal Ecology) and Prof Iza Radecka (Professor in Biotechnology)
For more information: For an informal discussion please email Dr Natasha Kruger - N.Kruger@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biological Sciences at
https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biological-sciences/
and click on Apply Now.
Biomedical Sciences Self-funded PhD projects (RIHS)
Reference: 5/PG-24/RIHS/BMSc
Self funded PhD Postgraduate research in Biomedical Sciences
Project details
Certain types of brain tumours develop much more aggressively and rapidly than others. Glioblastoma is a tumour that is known for its aggressive growth and resistance to treatment. Recent studies on glioblastoma biopsies have shown that many tumour cells have a reduced number of cilia (Sarkisian and Semple-Rowland, 2019). The primary cilia are microtubule organelles that project from the surface of cells (Bergmann, 2018) and have various functions including transportation of fluid into and out of the cell, epithelial structural functions, and sending and receiving signals into the cellular environment. Studies have shown that cilia are intimately involved in cell division therefore, it is possible that mutations that disrupt ciliogenesis could promote tumorigenesis as a result of a loss of cell cycle control (Plotnikova et al., 2008; Basten and Giles, 2013). Currently, it is largely unknown how the primary cilia are involved in these brain tumours and whether the cilia play a role in regulating the progression of cell growth.
The aim of the study is to determine whether the cilia are involved in these paediatric brain tumours and to establish whether there is a link between the stage of tumour progression and the stage of ciliogenesis dysregulation.
This research will investigate the rate of ciliogenesis in paediatric patient-derived tumour cells (GBM and ependymoma) using confocal microscopy. This will be compared to the number of cilia and rate of ciliogenesis with frozen sections of tumours from patient biopsy samples. Using RNA sequencing and qPCR analysis, we are planning to identify if there are any specific genes involved in the processes being studied and measure the expression levels of genes associated with dys-regulated pathways in ciliogenesis.
The results will provide an insight into the involvement of cilia in paediatric brain tumour cell proliferation and identify novel potential therapeutic targets for this type of malignancy.
Supervisory Team
- Goggolidou, Reader in Molecular Genetics, A. Karakoula, Senior Lecturer in Pharmacy
For more information: For an informal discussion please email Dr Goggolidou - p.goggolidou@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 12/IN-24/RIHS/BMSc
Self funded PhD Postgraduate research in Biomedical Sciences
Project details
There is clear epidemiological evidence that aspirin has a chemoprotective effect in colorectal cancer (CRC) development. Prophylactic aspirin use is thus currently considered when managing individuals that carry an identified mutation in highly penetrant genes that predispose them to familial CRC, including Lynch Syndrome (caused by mutations in DNA Mismatch Repair Genes) and Familial Adenomatous Polyposis (caused by mutations in the APC gene). As aspirin acts as a non-steroidal inflammatory (NSAID) agent, its effect on reducing inflammation is considered to be a primary mechanism for this observed chemoprotective effect. However, there is emerging evidence that aspirin and other NSAIDS can reduce tumour cell growth through additional mechanisms unrelated to inflammation. For example, Greenspan et al (2011), have indicated that NSAIDS including ibuprofen and aspirin can inhibit the activation of b-catenin - a key player in the Wnt signaling pathway that can drive cellular proliferation - by increasing b-catenin phosphorylation by a component of the APC destruction complex (GSK-3b) which is traditionally interpreted to enhance b-catenin degradation.
We have examined this phenomenon in preliminary experiments using confocal analysis and find an increased level of phosphorylated b-catenin at the centrosome in SW480 CRC cells incubated with aspirin – an unexpected result. b-catenin has additionally and traditionally been identified as a core component of the Zonula Adherens protein complex, playing a role in cell-cell adhesion and mechano-transduction bridging the cadherins and actin cytoskeleton (Farago et al, 2021). More recent experimentation is revealing that b-catenin processing at the centrosome may impact on the Wnt signaling pathway (Vora et al, 2020).
We thus wish to explore phosphorylated b-catenin localisation in CRC derived cell lines treated with a range of NSAIDS in detail, using immunochemical approaches including immunofluorescence analysis and confocal microscopy. We will additionally seek to isolate the centrosome using classical biochemical fractionation and identify interacting partners of phosphorylated b-catenin employing immunoprecipitation and mass spectrometry. This approach will provide insight into a) the molecular action of NSAIDS as anti-cancer agents and b) the centrosome-related function of b-catenin and is likely to result in high quality publications given the novelty of the experimental approach.
Farago, B., I.D. Nicholl et al, (2021) Activated nanoscale actin-binding domain motion in the catenin–cadherin complex revealed by neutron spin echo spectroscopy PNAS (USA) 118 (13) e2025012118; https://doi.org/10.1073/pnas.2025012118
Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW. Ibuprofen inhibits activation of nuclear {beta}-catenin in human colon adenomas and induces the phosphorylation of GSK-3{beta}. Cancer Prev Res (Phila). 2011 Jan;4(1):161-71. doi: 10.1158/1940-6207.CAPR-10-0021.
Vora SM, Fassler JS, Phillips BT. Centrosomes are required for proper β-catenin processing and Wnt response. Mol Biol Cell. 2020 Aug 1;31(17):1951-1961. doi: 10.1091/mbc.E20-02-0139.
Supervisory Team
Dr Iain D. Nicholl, DOS, Senior Lecturer in Biomedical Science
Dr Evi Goggolidou, Co-supervisor, Reader in Biomedical Science
For more information: For an informal discussion please email Dr Iain Nicholl - I.Nicholl@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 21/WW-24RIHS/BMSc
Self funded PhD Postgraduate research in Biomedical Sciences
Project details
Malignant mesothelioma (MM) is one of the most dreadful malignancies, which is commonly diagnosed at very late stage. The treatment outcomes in many other cancers have been significantly improved but the prognosis of MM remains very dismal. With most comprehensive treatment, MM patients can only survive for 9 – 12 months. Although it is a rare malignancy, the incidence of MM will significantly increase, especially in the developing nations. Due to the difficulty of getting rid of cancer tissues by surgery and the damaging effect of radiotherapy on the surrounding vital organs, chemotherapy becomes a major choice for MM treatment. Unfortunately, only two drugs (cisplatin and pemetrexed) are available for MM chemotherapy and MM cells are commonly resistant to all conventional anticancer drugs. Therefore, the current chemotherapy is more palliative rather than curative. The previous studies indicate that MM is heterogeneous containing cancer stem cells (CSCs). CSCs are highly invasive and resistant to conventional chemotherapies. CSCs are commonly located in poorly vascularised hypoxic regions. Tumour hypoxia is typically associated with chemo- and radio-resistance. Our previous study demonstrated that both hypoxia and NFκB pathway activation were co-detected in cells expressing CSC markers isolated from the core region of tumour spheres(1). High NFκB activity can be induced by hypoxia and detected in chemoresistant cancer, including MM(2, 3). Elucidation of the relationship between hypoxia, NFκB and CSCs may shed light on MM treatment.
The demand for novel anti-MM drug with low/non systemically toxic and highly efficacious is urgent but new drug development is a time (~15 years/drug) and cash ($1.5 billion/drug) consuming procedure. The repurposing of clinically available drugs into new treatment areas allows for the clinical development of safe compounds for new indications at much reduced time, risk and cost.
Disulfiram (DS), an antialcoholism drug without systemic toxicity to patients, shows very strong toxicity in CSCs from a wide range of cancer types including MM. In our pilot experiments, DS was highly toxic to MM cells and demonstrated curative effect on MM developed in mouse abdominal cavity. DS also blocked the expression of PD- L1, a protein compromising immune surveillance in MM patients. All of these findings indicate that DS is a very promising candidate for MM treatment. The studies from our and other groups indicate that the anticancer activity of disulfiram is copper (Cu) and zinc (Zn) dependent. DS can be promptly converted into diethyldithiocarbamate (DDC) which is a strong divalent metal ion chelator. DS chelates Cu(II) and Zn(II) forming a Cu-DDC or Zn-DDC complex which improves the transport of Cu and Zn into cancer cells. Cu-DDC and Zn-DDC complexes are strong reactive oxygen species (ROS) inducers. The ROS can also be induced by conventional anticancer drugs which are counterbalanced by ROS-activated NFκB which inhibits ROS-induced cytotoxicity and apoptosis. The Cu-DDC and Zn-DDC complex not only induces ROS it also inhibits NFκB activity in cancer cells(4, 5). It abolishes CSCs population in culture and completely reverses the chemoresistance and cross-resistance in chemoresistant cancer cells(3, 6, 7). Therefore, the functional anticancer compounds are Cu-DDC and Zn-DDC. This study intends to investigate the anti-MM activity of a novel injectable version of Cu-DDC and Zn-DDC, which is applicable in chest and abdominal cavities. We have developed PEGylated liposomal Cu-DDC and Zn-DDC (PEGlipo/Cu-DDC and PEGlipo/Zn-DDC) with promising drug loading contents and very stable shelf life in solution. The PEGlipo/Cu-DDC and PEGlipo/Zn-DDC showed very strong killing effect on other cancer cell lines. In this project, the effect of the new formulation of Cu-DDC and Zn-DDC on MM will be examined in cell culture and in mouse models. The molecular anticancer mechanisms, especially in CSCs and immune system, will be investigated.
Development of disulfiram derivatives in an anticancer setting will benefit MM patients and have significant financial implications for the NHS.
Relevant recent references
- P. Liu et al., Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget 5, 7471 - 7485 (2014).
- S. Liu, M. S. Wicha, Targeting breast cancer stem cells. J Clin Oncol 28, 4006-4012 (2009).
- P. Liu et al., Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. British journal of cancer, (2013).
- N. C. Yip et al., Disulfiram modulated ROS-MAPK and NFkB pathways and targeted breast cancer cells with cancer stem cell like properties. Br J Cancer 104, 1564-1574 (2011).
- X. Guo et al., Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer letters 290, 104-113 (2009).
- X. Guo et al., Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett 291, 104-113 (2010).
- P. Liu et al., Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer 107, 1488-1497 (2012).
Supervisory Team
Professor Weiguang Wang and Dr Vinodh Kannappans
For more information: For an informal discussion please email Professor Weiguang Wang at w.wang2@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 22/WW-24/RIHS/BMSc
Self funded PhD Postgraduate research in Biomedical Sciences
Project details
Pancreatic ductal adenocarcinoma (PDAC) is the 4th most common malignancy with very dismal prognosis. The findings indicate that PDAC are heterogeneous tumours containing cancer stem cells (CSCs). CSCs are highly metastatic and resistant to conventional chemotherapies becoming the primary source of PDAC relapse. CSCs are commonly located in poorly vascularised hypoxic regions. Tumour hypoxia is typically associated with chemo- and radio-resistance. Our previous study demonstrated that both hypoxia and NFκB pathway activation were co-detected in cells expressing CSC markers isolated from the core region of tumour spheres(1). High NFκB activity can be induced by hypoxia and detected in chemoresistant cancer(2, 3). It may also play a pivotal role in hypoxia-induced CSC characteristics and chemoresistance. Elucidation of the relationship between hypoxia, NFκB and CSCs may shed light on PDAC CSC-targeting.
Due to the time and costs for new drug development, repositioning of old drugs for new indications is an emerging drug R&D strategy in recent years. Disulfiram (DS), an anti-alcoholism drug used in clinic for over 60 years, demonstrates excellent activity against a wide range of cancers without toxicity to normal cells(1, 3-5). DS also potentiates the cytotoxic effect of anticancer drugs and increases the therapeutic index(4, 6). The anticancer effect of DS is copper (Cu) and zinc (Zn) dependent(7). Cu and Zn plays a crucial role in redox reactions and triggers the generation of reactive oxygen species (ROS) which induce apoptosis. The transport of Cu into the cell is strictly mediated by the trans-membrane Cu transporter Ctr1. DS can be promptly converted into diethyldithiocarbamate (DDC) which is a strong divalent metal ion chelator, DS chelates Cu(II) and Zn(II) forming a Cu-DDC or Zn-DDC complex which improves the transport of Cu and Zn into cancer cells. Cu-DDC and Zn-DDC complexes are much stronger ROS inducers than Cu and Zn alone. The ROS induced by conventional anticancer drugs is commonly counterbalanced by ROS-activated NFκB which inhibits ROS-induced cytotoxicity. The Cu-DDC and Zn-DDC complex not only induces ROS it also inhibits NFκB activity in cancer cells(5, 8). It abolishes CSCs population in culture and completely reverses the chemoresistance and cross-resistance in chemoresistant cancer cells(3, 4, 6). The data from Professor Wang’s lab shows that Cu-DDC and Zn-DDC block the sphere reforming activity and potentiates cytotoxicity of gemcitabine, paclitaxel and 5-fluorouracil in PDAC, while also blocking cancer invasion at very low concentrations. Although the anticancer activity of DS has been known for more than two decades, its use as a cancer treatment is limited when administered orally. The half-life of DS in the bloodstream is less than 4 minutes(7), which may explain the lack of encouraging results from several clinical trials (http://www.clinicaltrials.gov/) using oral administration. Nanotechnology-based drug delivery system (NDDS) is a rapidly evolving interdisciplinary field. Nanoparticles (NPs) can protect drugs from metabolism on their way to the cancer. We have successfully encapsulated DS into liposome, PLGA and gold NPs demonstrating improved anticancer efficacy in several mouse cancer models. These promising pilot data prompt us to develop biodegradable long-circulating NAB-encapsulated Cu-DDC and Zn-DDC to target PDAC CSCs.
The demand for novel anti-PDAC treatments is urgent while drug development is slow and costly, mainly due to the large risk of toxicity of novel molecules. The repurposing of clinically available drugs into new treatment areas allows for the clinical development of safe drugs for new indications at much reduced time, risk and cost. DS shows very promising anti-CSC activity in other types of cancer. This project aims to translate its derivatives, Cu-DDC and Zn-DDC into PDAC treatment.
- P. Liu et al., Liposome encapsulated Disulfiram inhibits NFκB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget 5, 7471 - 7485 (2014).
- S. Liu, M. S. Wicha, Targeting breast cancer stem cells. J Clin Oncol 28, 4006-4012 (2009).
- P. Liu et al., Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. British journal of cancer, (2013).
- P. Liu et al., Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer 107, 1488-1497 (2012).
- N. C. Yip et al., Disulfiram modulated ROS-MAPK and NFkB pathways and targeted breast cancer cells with cancer stem cell like properties. Br J Cancer 104, 1564-1574 (2011).
- X. Guo et al., Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett 291, 104-113 (2010).
- P. E. W. Tawari, Z.; Najlah, M.; Tsang, C. W.; Kannappan, V.; Liu, P.; McConville, C.; He, B.; Armesilla, A. L.; Wang, W., The cytotoxic mechanisms of disulfiram and copper(II) in cancer cells. Toxicology Research 4, 1439 - 1442 (2015).
- X. Guo et al., Disulfiram/copper complex inhibiting NFkappaB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer letters 290, 104-113 (2009).
Supervisory Team
Professor Weiguang Wang and Dr Vinodh Kannappans
For more information: For an informal discussion please email Professor Weiguang Wang at w.wang2@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 25/AE-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
Antimicrobial materials are essential in many medical applications including wound-healing. Due to their morphological similarity to the extra cellular matrix of skin, porous polymer scaffolds hold great potential for skin tissue engineering. Over the past couple of decades, metallic nanoparticles (e.g. gold and silver nanoparticles) have been extensively explored in wound-healing applications as efficient antimicrobial agents. Nevertheless, the use of metallic nanoparticles has raised concerns as these particles can penetrate into the stratum corneum of skin, or even diffuse into the cellular plasma membrane. Therefore, there is a real need for the development of polymer scaffolds with controlled release of metallic nanoparticles and preferably programmable release of different antimicrobial / anti-inflammatory agents as well.
In this project, we will utilise the three-dimensional (3D) structured emulsion-templated macroporous polymers (known as polyHIPEs) as biocompatible scaffolds to enable the incorporation of both traditional and metal based antimicrobial agents with programmable release. Our approach will allow the creation of customised antimicrobial 3D structures for a broad range of tissue engineering applications, with particular emphasis in wound-healing applications. The incorporation of a uniform, continuous layers of metallic nanoparticles/antimicrobial agents in the polyHIPE scaffolds will be verified by XPS analysis. Electron microscopy will be used to investigate morphological features of the scaffolds. The mechanical properties of the scaffolds will also be studies in details using compression/tensile testing and DMA. The antimicrobial efficacy of the antimicrobial scaffolds against a range of gram +ve and gram –ve bacterial e.g. (Staphylococcus aureus and Escherichia coli) will be determined by industry-standard AATCC protocols. Cytotoxicity analyses of the antimicrobial scaffolds toward human epidermal keratinocytes and human dermal fibroblasts will be performed using quantitative analyses of cell viability and proliferation.
In this project will involve close collaboration with microbiologists and biomedical scientists where investigations of the use of these antimicrobial scaffolds for skin tissue engineering / wound-healing applications will take place.
This project is part of ongoing research, so is suitable for either Chemistry, Microbiology or Biomedical Sciences PhD students as the direction of work can be adapted and shifted from one aspect to another with support from the team.
Relevant recent references
- A. M. Eissa, et al., “Reversible surface functionalisation of emulsion-templated porous polymers using dithiophenol maleimide functional macromolecules”, Chemical Communication 2017, 53, 9789 - 9792.
- C. Chen, A. M. Eissa, et al., "Emulsion-templated porous polymers prepared by thiol-ene and thiol-yne photopolymerisation using multifunctional acrylate and non-acrylate monomers", Polymer 2017, 126, 395–401.
- A. M. Eissa, et al., “Glycosylated nanoparticles as efficient antimicrobial delivery agents”, Biomacromolecules 2016, 17, 8, 2672-2679.
Supervisory Team
Dr Ahmed M. Eissa (Senior Lecturer); Professor Iza Radecka (Chair); Dr Leigh Jones (Senior Lecturer); Dr Abhishek Gupta (Senior Lecturer)
For more information: For an informal discussion please email to Dr Ahmed Eissa A.M.Eissa@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 26/AE-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
In this project, we will exploit the highly porous polymer scaffolds to create in vitro 3D tissue models. Recently, we have shown that emulsion-templated porous polymer scaffolds are capable of supporting 3D growth of many cell types including human pluripotent stem cells, human primary epithelial and stromal endometrial cells and human haematopoietic stem cells. Herein, we will broaden the scope of application of these scaffolds to address other biomedical problems including carcinoma by creating functional 3D cancer tumour models.
Polymerised high internal phase emulsions (polyHIPEs) will be first produced by emulsion templating whereby the continuous, oil-based phase of the emulsion is polymerised in the presence of the aqueous phase droplets, yielding a highly porous solid foam material. We will tailor the morphological and mechanical properties of our polyHIPE materials, making them more suitable for the culture of carcinoma tumours. We will also utilise different post-polymerisation approaches to functionalise our polyHIPE scaffolds by attaching interesting biomolecules/biomacromolecules that can facilitate notch signalling and therefore lays the foundation for biomimicry of the tissue niche. In collaboration with biomedical scientists and oncologists, the utility of the newly developed functional polyHIPE scaffolds will be fully characterised and exploited for the development of therapeutic strategies.
This a multidisciplinary project that involves development of the polymeric material and surface functionalisation with extracellular matrix proteins and/or polysaccharides as well as investigations of exploitation of its use for the development of cancer therapeutic strategies.
This project is part of ongoing research, so is suitable for either Chemistry or Biomedical Sciences PhD students as the direction of work can be adapted and shifted from one aspect to another with support from the team.
Relevant recent references
- C. E. Severn, A. M. Eissa, et al., “Ex vivo culture of adult CD34+ stem cells using functional highly porous polymer scaffolds to establish biomimicry of the bone marrow niche”, Biomaterials 2019, 225, 1195332.
- J. L. Ratcliffe, M. Walker, A. M. Eissa, et al., “Optimized peptide functionalization of thiol-acrylate emulsion-templated porous polymers leads to expansion of human pluripotent stem cells in 3D culture”, Journal of Polymer Science Part A: Polymer Chemistry 2019, 57, 1974.
- S. A. Richardson, et al., “Covalent Attachment of Fibronectin onto Emulsion‐Templated Porous Polymer Scaffolds Enhances Human Endometrial Stromal Cell Adhesion, Infiltration, and Function”, Macromolecular Bioscience 2019, 19(2), 1800351.
- A. M. Eissa, et al., “Reversible surface functionalisation of emulsion-templated porous polymers using dithiophenol maleimide functional macromolecules”, Chemical Communication 2017, 53, 9789 - 9792.
- C. Chen, A. M. Eissa, et al., "Emulsion-templated porous polymers prepared by thiol-ene and thiol-yne photopolymerisation using multifunctional acrylate and non-acrylate monomers", Polymer 2017, 126, 395–401.
- A. S. Hayward, A. M. Eissa, et al., “Galactose-functionalised polystyrene-based polyHIPE scaffolds for use in routine three dimensional culture of mammalian hepatocytes”, Biomacromolecules 2013, 14 (12), 4271–4277.
Supervisory Team
Dr Ahmed M. Eissa (Senior Lecturer); Dr Mark Morris (Reader); Professor Weiguang Wang (Chair); Dr Vinodh Kannappans (Senior Research Fellow)
For more information: For an informal discussion please email Dr Ahmed Eissa - A.M.Eissa@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 27/AE-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
|
Bioprinting is an emerging technology that enables the generation of precisely controlled 3D cell models and tissue constructs. Bioprinting process requires bioinks that contain living cells and biomaterials that mimic the extracellular matrix (ECM) environment; supporting cell adhesion, proliferation and differentiation after printing. There are many different biomaterials (natural or synthetic or a combination of the two as hybrid materials) reported as bioinks for 3D bioprinting. An ideal bioink should possess appropriate mechanical and biological properties of the target tissues and organs, which are essential to ensure correct functionality of the bioprinted tissues and organs. Hydrogels of natural polymers have been widely used in 3D bioprinting as they provide the desired microenvironment mimicking the native ECM for cell attachment and proliferation; however, they usually process poor shape fidelity and limited rigidity. While synthetic polymers may lack the ability to promote cellular adhesion when compared to natural polymers, they are promising candidates as they allow tuneable mechanical and morphological properties. Precursors of porous polymer scaffolds that recapitulate both mechanical and biochemical properties of the native ECM could potentially be utilised as a novel alternative to existing bioinks to generate functional bioprinted tissues. Porosity enables cell infiltration, tissue growth and vascularisation within the structural constructs. |
In this project we will utilise the bioprinting technology to develop novel hybrid scaffolds (hydrogels and macroporous polymers) to create an advanced ex vivo 3D tissue model in order to explore efficacy of novel drugs (e.g. anticancer). Recent work involved the synthesis of designed monomers and crosslinkers which can serve as a reactive ‘handle’ for subsequent chemical modification of the resulting biomaterials. The pore diameter and properties of materials can be tailored to a high extent, making them suitable for 3D cell culture, tissue engineering and regenerative medicine. Gelatine-based hydrogels will be synthesised and imbedded within the macroporous of polymer scaffolds to provide integrin-binding RGD motifs, promoting cell infiltration. Development of such novel hybrid polymer scaffolds will be trialed using modern polymerisation / chemical approaches.
This a multidisciplinary project that involves development of the polymeric material and optimisation of their morphological and mechanical characteristics as well as investigations of the use of these scaffolds in 3D cell culture and drug screening assays.
This project is part of ongoing research, so is suitable for either Chemistry or Biomedical Sciences PhD students as the direction of work can be adapted and shifted from one aspect to another with support from the team.
Relevant recent references
- S. A. Richardson, et al., “Covalent Attachment of Fibronectin onto Emulsion‐Templated Porous Polymer Scaffolds Enhances Human Endometrial Stromal Cell Adhesion, Infiltration, and Function”, Macromolecular Bioscience 2019, 19(2), 1800351.
- A. M. Eissa, et al., “Enhanced Differentiation Potential of Primary Human Endometrial Cells Cultured on 3D Scaffolds”, Biomacromolecules 2018, 19, 8, 3343-3350.
- A. S. Hayward, A. M. Eissa, et al., “Galactose-functionalised polystyrene-based polyHIPE scaffolds for use in routine three dimensional culture of mammalian hepatocytes”, Biomacromolecules 2013, 14 (12), 4271–4277.
Supervisory Team
Dr Ahmed M. Eissa (Senior Lecturer); Professor Weiguang Wang (Chair); Dr Mark Morris (Reader); Dr Vinodh Kannappans (Senior Research Fellow)
For more information: For an informal discussion please email Dr Ahmed Eissa - A.M.Eissa@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 41/OC-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
Cancer metabolomics to earlier diagnose gut and lung cancer in clinical and 3D biomaterials.
A hallmark of cancer progression is the fast metabolic reprogramming of cells in epithelial diseases such as gut and lung cancer. Yet, the 5-year prognosis for gut and lung cancer patients remains stubbornly low. The University of Wolverhampton’s Research Institute for Health Sciences (RIHS) has been successfully externally funded over many years to study the genetic basis of metastasis in brain cancers, from a genomics and proteomics perspective.
This project aims to develop a state-of-the-art metabolic profiling platform thereby extending the global analysis for small molecule prognostic markers for, and therapeutic targets against, epithelial cancers that may metastasise to the brain, ultimately resulting in clinical benefit.
Biomaterials (hydrogels and porous polymer materials) serving as scaffolds for 3D cell culture and tissue engineering are developed in Eissa’s group using modern synthetic chemistry and bioconjugation methods. The supervisory team’s recent work to build an in-house metabolomics database has shown that GC-MS and LC-HRMS technologies are capable of quantifying thousands of small molecule signatures for many diagnostic samples including human lung tissue and faecal samples from a myriad of gut diseases (Corcoran and Omar, 2024). The aim of the proposed work is to extend the 3D scaffold into fast-growing cancer cells to identify prognostic metabolic markers for, and therapeutic targets against, epithelial tumours that may metastasise to the gut, lung and brain.
This interdisciplinary project will create a range of complex architecture materials to serve as scaffolds for culturing gut and lung cells and, ultimately, tissue in 3D. This will involve progressive emulsion templating and additive manufacturing 3D printing technologies. One produced, accurately optimised and validated, scaffolds will be used to establish optimal in vitro tissue models of gut and lung cancers. Gold standard cancer drugs and novel plant-based natural products will be tested for anticancer potential. The outcome will be a robust analytical platform for investigating diverse cancer metabolic signatures in clinical and 3D in vitro models. This will have significant implications for RIHS, increasing the efficiency of the discovery process and translation of biomaterials and deliver a ‘step change’ in accelerating cancer therapy development.
Experience is required in subject areas including Chemistry, Immunology, Biomedical Science, Bioinformatics or a related field. This multidisciplinary project will involve working at the interface between materials chemistry and immunology. Prior experience is desirable but appropriate training in the full range of chemical and biomedical techniques will be provided to the successful candidate. Laboratory work will be in the Rosalind Franklin Building, which houses a broad range of state-of-the-art research facilities suitable for cell culture alongside recently installed state-of-the-art GC-MS and LC-HRMS. The project will involve collaborations with external research groups at the University of Warwick, Imperial College Department of Surgery & Cancer, and groups in Brazil, China and Mexico.
Applications are particularly welcomed from students of all backgrounds that are suitably qualified and highly motivated.
Supervisory Team
Director of Studies: Professor Olivia Corcoran, Senior Lecturer in Forensic Analysis, SOLS
Second Supervisor: Dr Hafid Omar, Reader in Immunology, RIHS
Third Supervisor: Dr Ahmed Eissa, Senior Lecturer in Organic Chemistry, SOLS
For more information: For an informal discussion please email Professor Olivia Corcoran o.corcoran@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 50/OO-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
Several studies have indicated the link between type 2 diabetes and Parkinson's disease (PD), and how one disease could lead to the development of the other. Specifically, it has been reported that oxidative stress associated with type 2 diabetes could lead death of brain cells observed in PD. Epidemiological studies have also indicated that 32% of people with type 2 diabetes will eventually develop PD. However, studies investigating how PD can contribute to the development of type 2 diabetes are lacking. PD is also associated with significant oxidative stress and the role that oxidative stress characterising PD plays in development of insulin resistance is not yet fully understood.
This project aims at investigating whether physiological changes associated with early stages of PD (including oxidative stress and differential expression of the alpha synuclein gene - a key biomarker for PD) could trigger metabolic changes observed in type 2 diabetes.
This project will employ cutting techniques in cellular and molecular biology to examine how oxidative stressors characterising PD affect metabolic processes in cells involved in glucose homeostasis (such as beta cells, adipocytes and muscle cells). Moreover, changes in glucose uptake and metabolism in cells transfected to over-express alpha synuclein gene will be studied. In addition, this project will observe changes in glucose homeostasis (and other parameters) in fruit fly model of PD in addition to protective effects of some novel incretin-based therapies.
Supervisory Team
Dr Opeolu Ojo (DOS), Dr Gavin McNee (Second Supervisor)
For more information: For an informal discussion please email Dr Opeolu Ojo o.ojo2@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 51/HO-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
Colorectal cancer (CRC) is a major cause of cancer-related mortality worldwide with an estimated 1.3 million cases diagnosed every year. Tumour bearing individuals are characterised by defects in immune mechanisms that normally eliminate incipient carcinoma. Growing evidence linked inflammation and CRC suggesting that pro-inflammatory microenvironment promotes tumour progression and invasion.
Inflammation is a complex process implicating the interaction of many inflammatory mediators. These include recruitment and activation of various inflammatory immune cells such as neutrophils, basophils, T cells, macrophages and dendritic cells (DC) as well as production of pro-inflammatory cytokines and mediators of inflammation. Interactions between all or some of these mediators can cause damage to the intestinal mucosa and may promote proliferation and metastasis of tumours.
Anaemia is associated with colorectal cancer but its treatment with oral supplementation of iron may prove inappropriate because the accumulation of iron in the intestinal tract can involve the generation of oxidative radicals and inflammatory mediators. This may also lead to infiltration of further inflammatory cells; in particular tumour associated macrophages (TAMs) promoting tumour progression as TAMs may provide tumours with iron rather than withholding it. Infiltration of DC may also play a major role in inducing inflammation. DC are the most potent antigen presenting cells that bridge the innate and adoptive immune systems and can initiate a cytotoxic immune response against cancer cells. However, the tumour may produce factors that induce DC migration to lymph nodes leading to tumour metastasis, especially at early stages of the disease. In addition, L-selectin facilitate the entry of T cells into secondary lymphoid tissues via high endothelial venules. However, we don’t know the effect of oral iron supplementation on DC recruitment into tumours or their migratory function.
Aims
The aim is to compare in normal and tumour tissues as well as serum from iron deficient patients who received either oral or systemic iron supplementation before surgery the following:
- Infiltration of immune cells and their phenotype in colorectal tumour tissues.
- Compartmentalisation of immunity and inflammatory markers in colorectal tumour tissues.
- Iron haemostasis related cytokines in tumour tissues and cytokine profile in the serum.
- Functional studies to investigate the effects of oral versus systemic iron supplementation on dendritic cell migration, T cell phenotype and activation and on Wnt signalling in peripheral blood mononuclear cells from healthy donors.
A wide range of techniques and protocols will be used in the course of this project including; RT-PCR, qPCR, ELISA, flow cytometry, luminex technology, immunohistochemistry and immunofluorescence microscopy.
Anticipated Outcome
The outcome may reveal whether oral iron supplementation is related to tumour development via changes in the immune profile either systemically or in the tumour microenvironment. The data could be harnessed to devise logical, evidence-based therapy targeting the innate and adaptive immune systems in colorectal cancer patients with iron deficiency anaemia.
Supervisory Team
Dr Omar Hafid, Dr Abhishek Gupta and Professor Matthew Brookes
For more information: For an informal discussion please email Dr Omar h.omar6@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Reference: 52/HO-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
The gastrointestinal tract is in contact with a huge variety of diverse pathogenic and commensal microbiota. Therefore, a balance between immunity and immune tolerance is required. Crohn’s disease (CD) is a chronic relapsing incurable inflammatory bowel disease (IBD) affecting 165/100000 people in the UK. The role of the gut microbiota is increasingly considered to be an important factor in the aetiology of IBD.
The cytokine milieu in the intestine is also an important factor in the maintenance of the immune balance and in IBD this balance is dysregulated resulting in mucosal inflammation. For example, in CD we found that the levels of proinflammatory cytokines are elevated, whereas levels of anti-inflammatory cytokines were reduced.
Dysbiosis or changes in bacterial diversity plays a major role in the progression of CD and associated with changes in the cytokine profile. While manipulating gut flora with probiotics has been effective in prevention of inflammation and maintenance of remission, probiotics are still ineffective in treating an established inflammation.
The application of biotherapeutics such as monoclonal antibodies against certain cytokines have revolutionised the treatment of various diseases including IBD. However, different gut bacteria may enhance different cytokine profile in the gut, particularly, during inflammatory conditions which may affect the efficacy of the biological drug.
Most investigations on the interaction between the gut immune system and the gut bacteria have been carried out on animal models and information on this interaction from human studies are sparse. In addition, we still do not fully know the effect of biological drugs on the bacterial composition and in relation to the systemic cytokine profile in CD patients.
Hence, we will investigate bacterial diversity and immune responses in patients with CD who are under biological treatment.
Aims
We aim to characterise the persistent dysbiosis in CD and examine concurrent changes in both gut flora and serum cytokine profiles in response to Infliximab.
Research Plan
Blood and stool samples will be obtained from IBD patients at three time points: before treatment, 6-8 weeks post treatment and 9 months post treatment. Blood and stool samples will also be obtained from healthy participants as a control group. Systemic TH1 and TH2 cytokines will be determined using Multiplex Bead-Based Immunoassay and flow cytometry techniques. Bacterial DNA will be extracted from the stool samples and the bacterial diversity will be assessed by 16s RNA gene sequence and microbial bioinformatic analysis.
The project will include a wide range of molecular biology and analytical techniques, including NGS, microbial DNA qPCR array, flow cytometry, immunofluorescence, Multiplex Bead-Based Immunoassay, 16S rRNA sequencing and bioinformatic analysis.
Anticipated outcome
Generated data will further our understanding of the bacterial composition/diversity in the human gut and whether changes in this diversity during inflammatory bowel disease is associated with a specific systemic cytokine profile. Results may also lead to optimisation of probiotic preparations for CD treatment.
Supervisory Team
Dr Omar Hafid, Dr Aikaterini Karakoula and Professor Matthew Brookes
For more information: For an informal discussion please email Dr Omar h.omar6@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Project: 54/AG-24/RIHS/BMSc
Self-funded PhD Postgraduate research in Biomedical Sciences
Project details
This project requires a candidate with interdisciplinary knowledge and/ or the commitment to explore beyond their previous experience.
This research endeavour presents a captivating opportunity to delve into revolutionary antimicrobial osteogenic biomaterials through Laser Powder Bed Fusion (L-PBF), marking a potential breakthrough in bone tissue engineering. By utilising the versatility of L-PBF technology and incorporating antimicrobial agents into osteogenic biomaterials, this study aims to meet the critical demand for personalised implants capable of both bone regeneration and innate resistance to microbial infections, especially crucial in orthopaedic procedures. The combination of osteogenic attributes with antimicrobial functionality will hold significant promise for customising implant materials and greatly enhancing patient outcomes.
The project materials innovation phase will identify emerging antimicrobial agents such as antimicrobial peptides, silver nanoparticles, or antibiotic-eluting polymers, and seek to embed them within osteogenic biomaterial matrices. Through rigorous experimentation, including the optimisation of L-PBF parameters and comprehensive characterisation, the research will aim to craft antimicrobial osteogenic biomaterials distinguished by heightened structural integrity, biocompatibility, and antimicrobial effectiveness. This pioneering approach will not only tackle current clinical challenges but also set the foundation for innovative strategies to combat implant-related infections, potentially diminishing the need for antibiotic therapy and revision surgeries.
Moreover, the exploration of antimicrobial osteogenic biomaterials via L-PBF will offer a compelling avenue for personalisation, facilitating the translation of research outcomes into practical applications tailored to individual patient needs. The opportunity to collaborate with clinical partners will provide invaluable insights into real-world challenges and further refine biomaterial development to match clinical requirements. The use of Finite Element Analysis (FEA) and Computational Fluid Dynamics (CFD) will enable the simulation of mechanical behaviour and fluid dynamics surrounding the fabricated implants under physiological conditions, providing crucial insights into their long-term functionality, and ensuring suitability for clinical use. Furthermore, this research will hold promise for commercialisation, with opportunities for patentable innovations. By bridging the gap between research and clinical practice, this ambitious project aims to advance both scientific understanding and patient care, driving innovation in bone tissue engineering and beyond.
Supervisory Team
Dr Abhishek Gupta, Senior Lecturer in Physiology and Pharmacology, Aaron Vance, Lecturer in Engineering, Professor Neil Ashwood, Consultant Orthopaedic Surgeon
For more information: For an informal discussion please contact via direct email to Dr Abhishek Gupta- a.gupta@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Biomedical Sciences at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-biomedical-sciences/
and click on Apply Now.
Chemistry Self-funded PhD Projects (RIHS)
Reference: 6/LJ-24/RIHS/Chemistry
Self funded PhD Postgraduate research in Chemistry
Project details
Recent work emanating from the Jones group has described the synthesis of the novel ligand “divan” (LH2) that upon Cu(II) metalation forms the complex [(MeCN)ÌCu(II)2(L)2] (1; Fig. 1) [1]. The two singly deprotonated L- ligands in 1 twist away from one another to form the dimeric Cu(II) structure. The distorted square planar metal geometries exhibit long apical Cu-NMeCN interactions formed through the accommodation of a guest MeCN molecule to give pseudo square based pyramidal topologies at both metal sites. Moreover, the guest MeCN has formed a formal Cu-N-Cu magnetic pathway in 1. This PhD project will explore further the chemistry of this prototype molecule as described below in the form of two work packages (WP1-2).
Figure 1 (a) Chemsketch of the ligand divan (LH2; R = Br). (b) Crystal structure of [(MeCN)ÌCu(II)2(L)2] as viewed perpendicular (b) and parallel (c) to the space-fill represented acetonitrile guest moiety. Colour code: Cu (green), C (grey), N (dark blue), O (red), Br (yellow). All hydrogen atoms have been omitted for clarity.
Work package 1: We will explore further the guest accommodating ability of this dimeric complex. Through careful guest selection we will be able to modulate and fine tune the resultant Cu(II)…Cu(II) magnetic exchange. Potential guests include (not exhaustive) pyrimidine (correct topology to forge 2 disparate Cu-Npyrimidine interactions); benzene / toluene (correct fit and would produce strong intermolecular interactions) and the azide (N3¯) anion can force ferromagnetic magnetic exchange. We will also investigate potential solution host-guest behaviour using NMR titration and / or UV-vis titration techniques [2].
Work package 2: Work would also focus on ligand modification towards molecular cavity modulation and therefore fine-tuning guest affinity with respect to the resultant host complex. Potential changes include Br replacement using Suzuki coupling transformations with (for instance) various commercially available boronic acid; [3] and the reduction of the imine C=N functional groups with NaBH4 or sodium triacetoxyborohydride (STAB) [4]. The initial change would potentially increase the molecular cavity size while the latter would significantly alter the ligand topology and would inevitably lead to a different complex topology upon metalation. In the same vein, we will also investigate the coordination ability of these novel ligands with other transition metals towards different magnetic behaviour. Likewise, diamagnetic Zn(II) analogues to 1 would be sought towards the aforementioned NMR titration studies.
The target material described here will be characterised via numerous techniques such as XRD (powder and single crystal); SQUID magnetometry and EPR (in conjunction with the University of Manchester). In summary, the successful execution of this project would give rise to a novel family of host-guest materials capable of hosting targeted guest molecules either towards their stabilization / sequestration or alternatively, fine-tuning magnetic exchange between the host magnetic metal centres.
References
- L. F. Jones et al. Unpublished results.
- P. Thordarson. Chem. Soc. Rev., 2011, 40, 1305-1323.
- C. C. C. Johansson Seechurn, M. O. Kitchin, T. J. Colacot and V. Snieckus. Angew. Chem. Int. Ed., 2012, 51, 5062-5085.
- M. B. Fugu, R. J. Ellaby, H. M. O’Connor, M. B. Pitak, W. Klooster, P. N. Horton, S. J. Coles, M. H. Al-mashhadani, I. F. Perepichka, E. K. Brechin, L. F. Jones. Dalton Trans., 2019, 48, 10180-10190.
Supervisory Team
Dr Leigh Jones (SL Chemistry) and Dr Ahmed Eissa (SL Chemistry)
For more information: For an informal discussion please email Dr Leigh Jones - Leigh.Jones@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Chemistry at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-chemistry/
and click on Apply Now.
Reference: 7/AE-24/RIHS/Chemistry
Self funded PhD Postgraduate research in Chemistry
Project details
The functionality, thermal stability, tuneable porosity and significant surface areas of both Metal-Organic Frameworks (MOFs)1 and Biopolymers2 has rendered them extremely promising hosts for the encapsulation (and immobilisation) of enzymes, thus allowing their use outside of the cell (Cell Free Enzymatic Catalysis). This project will combine the strengths of the PIs (Dr Leigh Jones: Metal-Organic Frameworks and Dr Ahmed Eissa: Biopolymers) towards the design and synthesis of novel host architectures (MOFs or biopolymers) that will then be employed to accommodate guest enzymes towards catalytic studies. Both MOF / Biopolymer surface adsorption as well as complete encapsulation of our target enzymes will be explored here. The target material described here will be characterised via numerous techniques such as XRD (powder and single crystal); SEM-EDX and TEM. All porous polymers will be assessed using (for instance) GC-MS and NMR studies.
In summary, the successful execution of this project would give rise to a novel family of heterogeneous host-guest enzyme catalysts. Furthermore, the candidate will glean vital experience in the fields of enzyme kinetics, coordination chemistry (MOF design, synthesis and characterisation) and biopolymer chemistry (e.g. porous polymer scaffolds).
References
(a) J. Mehta, N. Bhardwaj, S. K. Bhardwaj, K.-H. Kim and A. Deep. Coord. Chem. Rev. 2016, 322, 30-14. (b) X. Lian, Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang and H.-C. Zhou. Chem. Soc. Rev., 2017, 46, 3386—3401. (c) S. Huang, X. Kou, J. Shen, G. Chen and G. Ouyang. Angew. Chem. Int. Ed. 2020, 59, 8786 –8798. (d) N. Ye, X. Kou, J. Shen, S. Huang, G. Chen and G. Ouyang. ChemBioChem 2020, 21, 2585–2590. (e) X. Gao, Q. Zhai, M. Hu, S. Li and Y. Jiang. Catal. Sci. Technol., 2021, 11, 2446–2455.
- (a) K. M. L. Taylor-Pashow and J. G. Pribyl. 2019, 27, 1-26. (b) Muhammad Bilal and Hafiz M.N. Iqbal. Int. J. of Biological Macromolecules. 2019, 130, 462–482. (c) R. A. Wahab, N. Eliasa, F. Abdullaha and S. K. Ghoshal. Reactive and Functional Polymers. 2020, 152, 104613.
Supervisory Team
Dr Ahmed Eissa (SL, Chemistry) and Dr Leigh Jones (SL, Chemistry)
For more information: For an informal discussion please email Dr Ahmed Eissa - A.M.Eissa@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Chemistry at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-chemistry/
and click on Apply Now.
Reference: 8/LJ-24RIHS/Chemistry
Self funded PhD Postgraduate research in Chemistry
Post synthetic modification of Metal Organic Frameworks towards novel heterogeneous oxidative catalysts
Project details
In 2016, Dr Leigh Jones and his co-workers discovered that the monometallic complexes of general formula: [Mn(III)F3(H2O)(L)] (where L = 1,2-diimine ligand; Fig 1a and 1b) were extremely easy to synthesise in high purity and good yields (>65%) in a short space of time (< 5 mins).1 More recent unpublished results have shown that these complexes are able to catalyse the oxidation of trans-stilbene and the sulfoxidation of 4-nitrothioanisole and 4-nitrophenyl phenyl sulphide in competitive yields (75-95%).2
![Diagram illustrating crystal structures of [Mn(III)F3(H2O)(1,10-phen)] (a) and [Mn(III)F3(H2O)(2,2-bipy)] (b). (c) Schematic illustrating the potential binding sites within the MOF UiO-67-bipy](https://cdn-wlvacuk.terminalfour.net/media/departments/faculty-of-science-and-engineering/images/RIHS-diagram-452x162.jpg)
Figure 1 Crystal structures of [Mn(III)F3(H2O)(1,10-phen)] (a) and [Mn(III)F3(H2O)(2,2-bipy)] (b). (c) Schematic illustrating the potential binding sites within the MOF UiO-67-bipy.
This project will focus on using Post-Synthetic Modification (PSMet) techniques) to coordinate fluoride bound transition metal centres (starting with Mn(III) and Fe(III); both of which are commercially available) into the empty diimine sites located within the Metal-Organic Framework (MOF) UiO-67-bipy (Fig. 1c). Upon successful production of these novel materials ([TMF2/3(sol)x-bpy-UiO]), their ability to catalyse various oxidations (e.g. trans-stilbene) will be probed.3 The structure of the precursor MOF UiO-67-bipy comprises {Zr(IV)6} metal cluster nodes connected via linear 2,2¢-bipyridine-5,5¢-dicarboxylate ligands to give a porous extended architecture (BET surface area = 2277 m2 / g; pore size = 7.2 Å) along with the required uncoordinated bipyridyl sites required for metal ingression (as propose here). Indeed, Manna and co-workers have shown how Ir and Pd centres can be successfully integrated into bpy-UiO (with only small reductions in pore size) towards forging highly efficient heterogeneous catalysts.4
The target MOF material will be characterised using a number of techniques. Powder XRD will ascertain whether integration was successful upon comparison with the spectrum of the UiO-67-bipy precursor. ICP-MS, EDX and XRF will be employed to analyse the degree of TM loading (Zr:TM ratio). The extent of metal (TM / Zr host metal node) leaching post-reaction(s) will be monitored using ICP-MS, while p-XRD will assess MOF stability post use.
In summary, the successful execution of this project would mean that manganese and iron fluorides salts (among others) were effective and commercially cheap starting materials (w.r.t. rare earth metals such as Pd) in the production of novel heterogeneous MOF materials. It is worth stating that a plausible alternative / additional project direction would be to carry out H2 gas storage assessments upon production of such F- rich MOFs (driven by strong H…F hydrogen bonding interactions) using our newly acquired gas adsorption isotherm technology.
References
- E. Houton, B. Kelly, S. Sanz, E. J. L. McInnes, D. Collison, E. K. Brechin, A. G. Ryder, A.-L. Barra and L. F. Jones. Eur. J. Inorg. Chem., 2016, 32, 5123.
- C. Ratanasakaprakan, P. J. Murphy, D. Keddie, L. F. Jones. Unpublished Results.
- K. Hasan, N. Brown, C. M. Kozak. Green Chemistry. 2011, 13, 1230.
- K. Manna, T. Zhang and W. Lin. J. Am. Chem. Soc. 2014, 136, 6566.
Supervisory Team
Dr Leigh Jones (SL, Chemistry) and Dr Ahmed Eissa (SL, Chemistry)
For more information: For an informal discussion please email Dr Leigh Jones - Leigh.Jones@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Chemistry at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-chemistry/
and click on Apply Now.
Reference: 24/AE-24/RIHS/Chemistry
Self-funded PhD Postgraduate Research in Chemistry
Project details
The aim of this project is to develop synthetic multivalent ligand systems based on glycosylated polymersomes with tunable rigidity, permeability and size as a simple mimic of biological cells and a new delivery system for bioactive molecules.
Polymer vesicles (polymersomes) are spherical soft-matter (nano)capsules consisting of a bilayer membrane enclosing an aqueous compartment and are generally formed by spontaneous self-organisation from amphiphilic block copolymers. Compared to lipid vesicles (liposomes), they have a relatively thick and robust membrane formed by polymeric amphiphiles with a relatively high molecular weight, which can increase their biological stability and prolong the circulation time in blood. Furthermore, polymersomes can present biologically active functionalities, such as sugars, on their external surface by self-assembly of functionalised amphiphilic polymers. Many biological processes in mammalian cells, such as fertilisation, viral and microbial infections, inflammation and cancer cell metastasis, are mediated by sugar-protein (lectin) interactions. Multivalent glycosylated macromolecules (glycopolymers) bind to lectins with high avidity and so provide an attractive therapeutic strategy for tackling diseases that involve sugar-lectin binding during disease progression. Moreover, these sugar molecules through interaction with cell-surface lectins can promote uptake of nanoscale particles by cells (e.g. galactose binds specifically to liver cells that possess high levels of the receptor ASGP-R). Sugar-decorated polymersomes (glycopolymersomes) therefore hold great promise in nanomedicine as vectors for targeted delivery and a simple model of biological cells.
In this project, we will synthesise well-defined amphiphilic block glycopolymers using the recently developed photo-induced reversible deactivation radical polymerisation and study their self-assembly, by different methods, into both small and giant unilamellar vesicles. Molecular characterisation will be performed by NMR, MALDI-tof MS and GPC, and extensive studies of the aqueous solution behaviour of these polymers (solubilities, cloud point, critical aggregation concentration, dynamic light scattering (DLS) and transmission electron microscopy (TEM)) will also be performed. Loading of the vesicles with model compounds and therapeutics, as well as studies of the membrane permeability will be explored.
This project is part of ongoing research, so is suitable for either Chemistry or Biomedical Sciences PhD students as the direction of work can be adapted and shifted from one aspect to another with support from the team.
Relevant recent references
- Y. Li, Y. Chang, D. M. Haddleton, N. R. Cameron and A. M. Eissa “Comprehensive Glycoscience 2nd edition, Volume 4: Glyconanotechnology, Chapter 00114. Glycopolymer Functionalized Nanoparticles and Their Applications” by the Elsevier 2021, 209-249.
- A. R. Hall, J. T. Blakeman, A. M. Eissa, et al., “Glycan–glycan interactions determine Leishmania attachment to the midgut of permissive sand fly vectors”, Chemical Science 2020,11, 10973-10983.
- L. Martin, et al., “Polydimethylsiloxane-Based Giant Glycosylated Polymersomes with Tunable Bacterial Affinity”, Biomacromolecules 2019, 20, 3, 1297-1307.
- Y. Luo, et al., “Synthesis of glycopolymers with specificity for bacterial strains via bacteria-guided polymerization”, Chemical Science, 2019, 10, 5251-5257.
- J. Binfield, et al., “Imaging Proton Transport in Giant Vesicles through Cyclic Peptide–Polymer Conjugate Nanotube Transmembrane Ion Channels”, Macromolecular Rapid Communications 2018, doi.org/10.1002/marc.201700831.
- A. Kubilis, et al., “Giant polymersome protocells dock with virus particle mimics via multivalent glycan-lectin interactions”, Scientific Reports 2016, 6, 32414.
- A. M. Eissa, et al., “Glycosylated nanoparticles as efficient antimicrobial delivery agents”, Biomacromolecules 2016, 17, 8, 2672-2679.
- A. M. Eissa, et al., “Polymersome‐forming amphiphilic glycosylated polymers: Synthesis and characterization”, J. Polym. Sci. Polym. Chem. 2013, 51(24), 5184-5193.
Supervisory Team
Dr Ahmed M. Eissa (Senior Lecturer); Dr Leigh Jones (Senior Lecturer); Professor Iza Radecka (Chair)
For more information: For an informal discussion please email Dr Ahmed Eissa - A.M.Eissa@wlv.ac.uk
Apply online for the PhD Postgraduate research in Chemistry at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-chemistry/
and click on Apply Now.
Molecular Biology Self-funded PhD Projects (RIHS)
Reference: 4/AG-24/RIHS/MolecBio
Self funded PhD Postgraduate research in Molecular Biology
Project details
Glioblastoma remains as the most common and aggressive malignant brain tumour, standing with a poor prognosis and treatment prospective. Despite the aggressive standard care, such as surgical resection and chemoradiation, median survival rates are low; about 15 months from the diagnosis and a 5-year survival rate of only 5%. Treatment resistance arises from a wide variety of mechanisms, including the blood–brain barrier (BBB), inter- and intra-tumoral heterogeneity, and a profoundly immunosuppressive tumour microenvironment.
Curcumin (CUR), chemically known as diferuloylmethane, obtained from Curcuma longa plant, is a polyphenolic compound that is well known for its pharmacological benefits like, antitumor, anti-inflammatory, and antioxidant. The anticancer properties of CUR are attributed to its unique abilities of inducing apoptosis and inhibiting proliferation and invasion of tumours by suppressing cellular signalling pathways in various cancers, including malignant gliomas. Furthermore, several studies have demonstrated that CUR is able to cross the BBB as well as to interact synergistically with commonly used chemotherapeutics making it an attractive therapeutic agent for malignant brain tumours. However, the biomedical application of CUR is greatly hindered by its hydrophobicity and low bioavailability. The poor bioavailability of CUR might be associated with its poor absorption, quick metabolism and rapid systemic elimination. Several research attempts have been made to produce structural modifications in CUR molecule to enhance its solubility and selective toxicity towards cancer cells. Moreover, different delivery systems have been produced to improve its physiochemical properties.
Microencapsulation of medicinal substances in a suitable carrier is a common practice in pharmaceutics for drug delivery. Cyclodextrins (CDs) are naturally occurring cyclic oligosaccharide obtained from starch by enzymatic cyclisation that are used as pharmaceutical adjuvants. Our group has reported the production of water-soluble CUR inclusion complex with hydroxypropyl-β cyclodextrins (HPβCD) by solvent evaporation method. CUR:HPβCD has demonstrated reduced viability (in vitro) against the range of tested cancer cell lines demonstrating that CUR maintains its anticancer properties in the inclusion complex.
Techniques associated with this project
The current project will involve the production of a CUR:HPβCD complex and evaluating its anticancer properties against malignant glioma cells using a wide range of cellular and molecular biology/genetics techniques.
Key references
- Gupta, et al., 2019. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. European Polymer Journal 118: 437-450
Giordano A. and Tommonaro G, 2019. Curcumin and Cancer. Nutrients, 11:2376.
Mansouri, K. et al., 2020. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer, 20: 791.
Perry M. et al., 2010. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res, 54: 1192–1201.
Purkayastha S. et al., 2009. Curcumin blocks brain tumor formation. Brain Res, 1266: 130–138.
Ramachandran C. et al., 2012. Potentiation of etoposide and temozolomide cytotoxicity by curcumin and turmeric force™ in brain tumor cell lines. J Complement Integr Med, 9: article 20.
Weissenberger J. et al., 2010. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res, 16: 5781–5795.
Zanotto-Filho A. et al., 2013. Curcumin-loaded lipid-core nanocapsules as a strategy to improve pharmacological efficacy of curcumin in glioma treatment. Eur J Pharm Biopharm, 83: 156–167.
Zanotto-Filho A. et al., 2015. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett, 358: 220–231.
Zhuang W. et al., 2012. Curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Cancer Sci, 103: 684–690.
Supervisory Team
Dr Abhishek Gupta and Dr Aikaterini Karakoula
For more information: For an informal discussion please email Dr Abhishek Gupta a.gupta@wlv.ac.uk and/or Dr Aikaterini Karakoula a.karakoula@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Molecular Biology at
https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-molecular-biology/
and click on Apply Now.
Reference: 10/OH-24/RIHS/MolecBio
Self funded PhD Postgraduate research in Molecular Biology
Project details
Glioblastoma remains as the most common and aggressive malignant brain tumour, standing with a poor prognosis and treatment prospective. Despite the aggressive standard care, such as surgical resection and chemoradiation, median survival rates are low. Treatment resistance arises from a wide variety of mechanisms, including the blood–brain barrier (BBB), inter- and intra-tumoral heterogeneity, and a profoundly immunosuppressive tumour microenvironment.
Metabolic adaptation processes are believed to provide cancer cells, including glioblastoma, with proliferation and survival benefits over normal cells. However, these processes can also make cancer cells selectively dependent or addicted to certain nutrients and metabolic pathways. The overlapping metabolic reprogramming of cancer and immune cells is a putative determinant of the antitumor immune response in cancer. Increased evidence suggests that cancer metabolism not only plays a crucial role in cancer signalling for sustaining tumorigenesis and survival, but also has wider implications in the regulation of antitumor immune response through both the release of metabolites and affecting the expression of immune molecules.
Recent work in our lab has confirmed that overexpression of a key glycolytic enzyme hexokinase 2 (HK2) is associated with a shorter overall survival in glioblastoma patients. HK2 is an important facilitator of aerobic glycolysis in GBM, enabling survival and proliferation of the tumour microenvironment. A significant decrease in cell proliferation was established through CRISPR-mediated knockout of the HK2 gene in our patient-derived glioblastoma cell cultures, suggesting the potential of HK2 as a therapeutic target in glioblastoma patients. Results from CRISPR-treated glioblastoma cultures using RNA-sequencing analysis revealed significant deregulated immune-related signalling pathways including Toll-like receptors (TLR1, TLR2, TLR4, TLR5, TRL6), immune-checkpoint inhibitors (HAVCR2 and PDCD1LG2), JAK-STAT signalling pathway (STA1, 2, 4, 5A, 6), cytokine-mediated signalling and response to inflammation (IL6R, IL6ST). Activation of TLRs indicates an inflammatory profile within the tumour microenvironment. This can lead to the recruitment of inflammatory immune cells and the production of proinflammatory cytokines, chemokines and growth factors which can promote tumour development and progression. Reduction in glioblastoma low-grade inflammation may render tumour cells susceptible to cancer drugs and anti-tumour immune responses.
More recently, attention has been given to the impact of alterations in glycolytic pathways on not only proliferating tumour cells but also the tumour microenvironment and resulting changes in immune cell metabolism and function. Metabolic reprogramming within tumour cells diminishes the function of effector immune cells through depletion of essential metabolites and promotes enrichment of suppressive immune populations.
In this 3-year research project, we plan to further explore the effects of GBM aerobic glycolysis and immunosuppression through HK2 to identify potential therapeutic targets and pathway interactions within the heterogeneous tumour microenvironment. We also plan to assess the inflammatory profile within the glioblastoma microenvironment by investigating the production of proinflammatory as well as Th2 type cytokines and chemokines that signal via the JAK-STAT pathway. This project will provide extensive training in a wide range of cell and molecular biology, and analytical techniques, including but not limited to RT2 Profiler PCR Arrays, flow cytometry, confocal, immunofluorescence and Luminex technology.
Key references:
Bi J., Bi F., Pan X., Yang Q. (2021) Establishment of a novel glycolysis-related prognostic gene signature for ovarian cancer and its relationships with immune infiltration of the tumor microenvironment. J. Transl. Med., 19:382.
Coussens L.M., Werb Z. (2022). Inflammation and cancer. Nature, 420(6917):860-867.
Choudhary N., Osorio R.C., Oh J.Y., Aghi M.K. (2023). Metabolic Barriers to Glioblastoma Immunotherapy. Cancers 15(5):1519.
DePeaux K., Delgoffe G.M. (2021). Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21:785–797.
Jarmuzek P. et al. (2023) Cytokine Profile in Development of Glioblastoma in Relation to Healthy Individuals. Int J Mol Sci. 24(22):16206.
Muir A., Vander Heiden M.G. (2018). The nutrient environment affects therapy. Science, 360:962–963.
Ni Y., Low J.T., Silke J., O'Reilly L.A. (2022). Digesting the Role of JAK-STAT and Cytokine Signaling in Oral and Gastric Cancers. Front Immunol.,13:835997.
Supervisory Team
Dr Omar Hafid and Dr Aikaterini Karakoula
For more information: For an informal discussion please email Dr Aikaterini Karakoula A.Karakoula@wlv.ac.uk and/or Dr Hafid Omar H.Omar6@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Molecular Biology at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-molecular-biology/
and click on Apply Now.
Pharmacy Self-funded PhD Project
Botany Self-funded PhD Project
Reference: 9/AG-24/Pharmacy
Self funded PhD Postgraduate research in Pharmacy
Project details
Wound healing a complex physiological process involving several stages. The completeness and length of time to resolution depends on whether the wound is acute or chronic. Market research analysis has reported the current (2024) advanced wound care market values at US $11.66 billion which is expected to rise to US$16.12 billion by 2034 (Advanced Wound Care market). This is due to the rise in prevalence of chronic wound disorders in high-risk population like diabetics, elderly, and immunocompromised patients. There is a plethora of wound care products, yet the pervasiveness of chronic wounds is on the rise, highlighting the ongoing necessity for the development of efficacious wound dressings.
Mother nature has always been an excellent source for potent compounds with healing properties. With an objective to enrich patients' quality of life and mitigate the socioeconomic impact associated with chronic wounds, our group has been working on biosynthetic hydrogels for wound management (Gupta et al., 2016, 2019, 2021). To further improve healing process, selected lipids can be introduced to the topical formulations/hydrogels due to their promising moisturising properties (Gope et al., 2022, de Albuquerque et al., 2023). In addition, the immergence of antibiotic resistant microbial strains necessitated search for newer alternative antimicrobial agents. The emergence of nanotechnology, enabling the production of metal nanoparticles, has served a new therapeutic modality. Attributing to their characteristic antimicrobial properties, metal nanoparticles have received increased interest in biomedical applications.
The current project will underpin the development of advanced lipid-based materials infused with natural healing agents for wound dressing applications. A range of lipids, from animal and vegetable sources, together with selected metal nanoparticles will be investigated.
The antimicrobial and associated healing properties of nanoparticles and lipid will be evaluated both individually and in synergy. The results of the study will determine whether nanoparticles will be blended with the selected lipid and incorporated into the biosynthetic matrix for potential wound dressing application, either combined or loaded separately. Various methods for blending lipids with nanoparticles may be investigated. Once the potent material is produced, a range of physicochemical and biological characterisation studies will be undertaken to evaluate the suitability of these materials for wound management as dressings. These methods will include the assessment of the chemokine and the cytokine profiles of immune cells associated with wound healing using Luminex technology based on cytokine/chemokine multiplex techniques. Functional studies will also be carried out to determine the effects of nanoparticles-lipid complex on the capacity of immune cell migration and recruitment.
References
Advanced Wound Care Market: https://www.factmr.com/report/4822/advance-wound-care-market
de Albuquerque, P.B.S. et al., 2023. The Use of Proteins, Lipids, and Carbohydrates in the Management of Wounds. Molecules. 28: 1580. doi: 10.3390/molecules28041580.
Gope, A. et al., 2022. Regenerative repair of full thickness skin wound assisted by dual crosslinking percolative gel casting maneuvered alginate hydrogel embedded with honey ghee blend resembles standard cutaneous properties, Journal of Tissue Viability,31, pp. 657-672, https://doi.org/10.1016/j.jtv.2022.07.007.
Gupta, A. et al., 2016. Characterisation and in vitro antimicrobial activity of biosynthetic silver-loaded bacterial cellulose hydrogels. Journal of Microencapsulation, 33, pp. 725-734.
Gupta, A. et al., 2019. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in Cyclodextrins as wound dressings. European Polymer Journal, 118, pp. 437-450.
Gupta, A. et al., 2020. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose Based Hydrogels for Wound Dressing Applications. BioMacromolecules, 21, pp. 1802–1811.
Mohamed, D.S. et al., 2020. Antimicrobial Activity of Silver-Treated Bacteria against other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics, 9, 181. https://doi.org/10.3390/antibiotics9040181.
Supervisory Team
Dr Abhishek Gupta, Prof Izabela Radecka and Dr Hafid Omar
For more information: For an informal discussion please email Dr Abhishek Gupta - a.gupta@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Pharmacy at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-pharmacy/
and click on Apply Now.
Reference: 56/KK-24/Pharmacy
Self funded PhD Postgraduate research in Pharmacy
About the Project:
- Problem
- Testing/methodology
- Solution/output
Glioblastoma is a malignant glioma associated with a mean overall survival of 12 to 18 months, even with optimal treatment, due to its high relapse rate and resistance to current treatments [1]. The major limitations of currently used therapies are crossing the blood-brain barrier (BBB) that hinders drugs from reaching the target tumour glioblastoma cells [2]. Nanotherapeutics hold a promising attribute in solving various limitations of conventional drug delivery having low profile in bio-distribution, targeting, solubility and therapeutic index. Attributing to high surface-to-volume ratio, nanocarriers are favoured for therapeutic delivery over traditional methods, which enables the rapid release of drugs to targeted areas [3]. Recent studies specifically evaluated the systematic targeting of glioblastoma using nanoparticles and their capacity to selectively deliver therapies to target cells [4-6].
Metal nanoparticles are commonly synthesised from noble metals like copper, silver, gold and platinum. Among these, gold nanoparticles (AuNPs) have garnered attention for their advantageous roles in biomedical research [7]. Use of phytochemical compounds in medicine is regaining popularity. Preclinical investigations have demonstrated curcumin (CUR), a phytochemical polyphenol compound derived from Curcuma longa, having anticancer effects on glioma, including the reduction of cell proliferation, migration, and invasion, along with the promotion of apoptosis and autophagy in glioma cells. Additionally, curcumin is able to cross the BBB and has demonstrated antiangiogenetic effect [8,9]. Furthermore, CUR is known to reduce silver and gold hence, used for nanoparticle synthesis [7].
In the current project, CUR reduced AuNPs will be produced and their delivery through the BBB, for therapeutic effect, will be tailored by using solubility enhancing carriers. The range of solubility enhancing carriers like cyclodextrins and liposomes will be employed to achieve high drug delivery through the BBB.
The study will be extended by synthesising poly(amidoamine) (PAMAM) dendrimers encapsulated gold (PAMAM-Au) nanoparticles within the diameter below 10 nm [10]. These carriers can not only cross the BBB but also aid in imaging by enhancing the radiation absorption capacity of tumour cells [11]. Using these gold nanoparticles as a vehicle, targeted delivery of anti-neurodegenerative cancer drug; LMP 400(Indotecan) can be achieved by functionalising the Indotecan/PAMAM-Au conjugate with anti-EZH2 antibody. EZH2 protein overexpression is observed in most neurodegenerative diseases including glioblastomas thus can be used as a biomarker [12-13].
To achieve the binding of indotecan with the PAMAM dendrimers, surface functionalisation of PAMAM dendrimers with PEG-OH is achieved [14], this will not only help in crossing the blood-brain barrier with ease but also help in conjugation with of dendrimers with drug and anti-EZH2 antibody; both have carbonyl groups on their surface that can show nucleophilic reaction with hydroxyl groups of PEG-OH functionalised dendrimers. Characterisation techniques such as; SEM, TEM, AFM, XRD, zeta potential, drug loading, and drug release studies are all carried out to assess the validity of the study [15]. This study is organised to achieve maximum therapeutic output at specific glioblastoma cells by crossing the blood-brain barrier with the help of most biocompatible and theragnostic gold nanoparticles.
References:
- Alphandery, E. Nano-Therapies for Glioblastoma Treatment. Cancers, 2020, 12, 242.
- Zhou, Y., et al., Glioblastoma cell-derived exosomes functionalized with peptides as efficient nanocarriers for synergistic chemotherapy of glioblastoma with improved biosafety.Nano Research, 2023, 16, 13283-13293.
- Allen NC, et al., Optimization of Tumor Targeting Gold Nanoparticles for Glioblastoma Applications. Nanomaterials (Basel), 2022, 12, 3869.
- Gregory, J.V., al., Systemic brain tumour delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun., 2020, 11, 5687.
- Alghamri, M.S., et al., Systemic delivery of an adjuvant CXCR4-CXCL12 signaling inhibitor encapsulated in synthetic protein nanoparticles for glioma immunotherapy. ACS Nano, 2022, 16, 8729-8750.
- Coluccia, D., et al., Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine, 2018, 14, 1137-1148.
- Muniyappan, N., Pnadeeswaran, M. and Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti- inflammatory activities. Environmental Chemistry and Ecotoxicology 2021, 3, 117-124.
- Luís, Â.; Amaral, L.; Domingues, F.; Pereira, L.; Cascalheira, J.F. Action of Curcumin on Glioblastoma Growth: A Systematic Review with Meta-Analysis of Animal Model Studies. Biomedicines, 2024, 12, 268.
- Beylerli, O.; Beilerli, A.; Shumadalova, A.; Wang, X.; Yang, M.; Sun, H.; Teng, L. Therapeutic effect of natural polyphenols against glioblastoma. Cell Dev. Biol. 2022, 10, 1036809.
- Kim, Y.-G., S.-K. Oh, and R.M. Crooks. Preparation and Characterization of 1−2 nm Dendrimer-Encapsulated Gold Nanoparticles Having Very Narrow Size Distributions.Chemistry of Materials, 2004, 16(1), 167-172.
- Gao, Q., et al., Gold Nanoparticles in Cancer Theragnostic.Frontiers in Bioengineering and Biotechnology, 2021, 9
- de Vries, Nienke A., et al., Prolonged Ezh2 Depletion in Glioblastoma Causes a Robust Switch in Cell Fate Resulting in Tumor Progression.Cell Reports, 2015, 10(3), 383-397.
- Alshora, D.H., M.A. Ibrahim, and F.K. Alanazi, Chapter 6 - Nanotechnology from particle size reduction to enhancing aqueous solubility, in Surface Chemistry of Nanobiomaterials, A.M. Grumezescu, Editor. 2016, William Andrew Publishing. p. 163-191.
- Zhang, F., et al., Uniform brain tumor distribution and tumor-associated macrophage targeting of systemically administered dendrimers.Biomaterials, 2015, 52, 507-16.
- Jain, A.K. and S. Thareja. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery.Artificial Cells, Nanomedicine, and Biotechnology, 2019, 47(1), 524-539.
Supervisory Team
Second Supervisor: Dr Abishek Gupta, Director of Studies: Dr Katherine Karakoula
For more information: For an informal discussion please email Dr Katherine Karakoula a.karakoula@wlv.ac.uk
Reference: 3/TB-24/RIHS/Botany
Self funded PhD Postgraduate research in Botany
Project details
Tropical montane rain forests are biodiversity hotspots, uniquely threatened by climate change and deforestation. Characterised by cool temperatures, high humidity and periodic or persistent cloud immersion, these ecosystems host many endemic species specialised for life in high moisture environments. As the climate warms, cloud bases will rise, and the duration of cloud immersion will consequently decrease. The impact of this climate disruption is often exacerbated by forest fragmentation, resulting in extreme risk of extinction for many species endemic to this environment.
Current evidence suggests that declining humidity and the loss of cloud immersion will strongly affect epiphytes, particularly drought-sensitive taxa. Moreover, little is known of the pollination syndromes and breeding systems of the majority of these species. Many are poorly recorded, and often still unidentified. One such genus of epiphytes, is the Andean orchid genus Restrepia, which the supervisory team (Dr Baldwin and Dr Bachman) have studied for many years.
Considering the issues described above, the objectives of this project will be to undertake the first, International Union for the Conservation of Nature (IUCN) validated, study of current threats posed to Restrepia and trends in extinction risk over time (Red List status and Index) this will be performed in collaboration with the Royal Botanic Gardens, Kew under the supervision of Dr Bachman. In conjunction with the Red Listing component of the proposed study, a detailed analysis of the floral ultrastructure of a model species (R. brachypus) using both light and transmission electron microscopy (supervised by Dr Baldwin at the University of Wolverhampton), will be performed, to further our understanding of the reproductive biology of these plants, which is crucial to the proposed analysis of the continued genetic viability of the genus.
The expected outputs for this project will result include the completion of Red List assessments for all 61 species of Restrepia and the identification of the major threat drivers and conservation actions required. In addition, trends in extinction risk over time will be charted, using the Red List Index covering the past three decades. Assessments will be submitted for publication on the IUCN Red List with a supporting dataset of digitised and georeferenced Restrepia occurrences. In conjunction with a better understanding of the reproductive biology of these species, the published Red List assessments will underpin potential conservation interventions made through regional (e.g. Colombia Plant Specialist Group) and international agencies (e.g. IUCN). As well as publication on the IUCN Red List, knowledge dissemination of the outputs of this research will occur via research conferences and publication in open access journals, with the broadest possible reach.
Supervisory Team
Dr Timothy C. Baldwin (Reader in Plant Cell Biology)
Dr Steven Bachman (Royal Botanic Gardens, Kew)
For more information:
For an informal discussion please email Dr Timothy Baldwin - T.Baldwin@wlv.ac.uk
Apply online
Apply online for the PhD Postgraduate research in Botany at https://www.wlv.ac.uk/courses/phd-postgraduate-research-in-botany/
and click on Apply Now.


/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/Diane-Spencer-(Teaser-image).jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-18-19/220325-Engineers_teach_thumbail.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240509-Menopause-Research-Resized.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images/Maria-Serria-(teaser-image).jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/241014-Cyber4ME-Project-Resized.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/240315-Research-Resized.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/BDA-group-photo.jpg)





